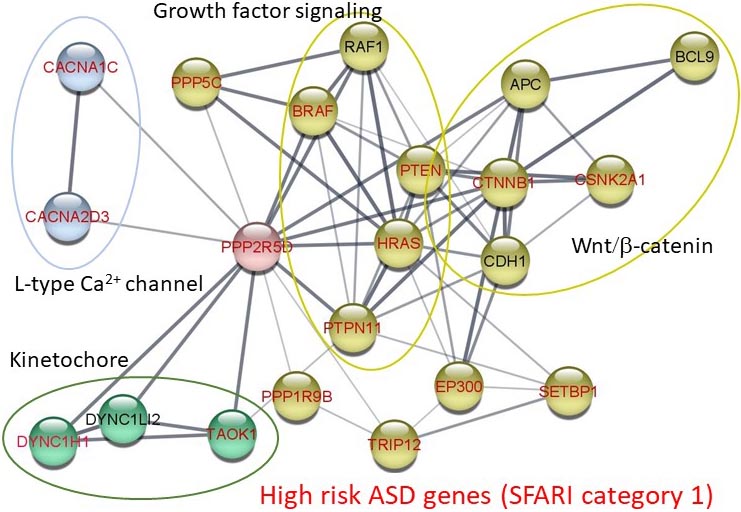

Houge-Janssens Syndrome 1 or Jordan’s Syndrome (JS) is a highly penetrant neurodevelopmental disorder caused by recurrent, mostly de novo mutations in PPP2R5D, the gene encoding B’delta (a.k.a. B56delta). PPP2R5D is also a category 1 autism risk gene according to the Simons Foundation's SFARI and interacts physically and functionally with other category 1 autism risk genes.
B’delta is one of 12 regulatory subunit of protein phosphatase 2A (PP2A). Like other PP2A regulatory subunits, B’delta is thought to confer to the PP2A holoenzyme substrate specificity/activity, subcellular localization, and regulation by second messengers. However, little is known how wild-type B’delta contributes to PP2A function, let alone how B’delta (and not other regulatory subunit) mutations cause JS. Compounding the plight of JS children and their caregivers is that many of the same mutations in PPP2R5D have recently been associated with early-onset Parkinsonism.
The lab seek to fill in these critical knowledge gaps by addressing the following questions:
What are the signaling cascades and phospho-substrates impacted by PP2A mutations that derail normal brain development?
What are the developmental stages, brain regions, and cell types involved in the manifestation of neurological phenotypes of JS?
Is it possible to treat the symptoms of JS with drugs?
To answer these questions, we have made both germline and Cre-inducible mouse models of JS, which according to preliminary evidence faithfully recapitulate the human condition. We have also used drugs that are close to FDA approval to rescue learning and memory deficits in JS-model model mice. Because PPP2R5D mutations are also implicated in neurodegenerative, and -psychiatric conditions, our research has implications beyond monogenic neurodevelopmental disorders.