Breadcrumb
PKA in neurological disorders
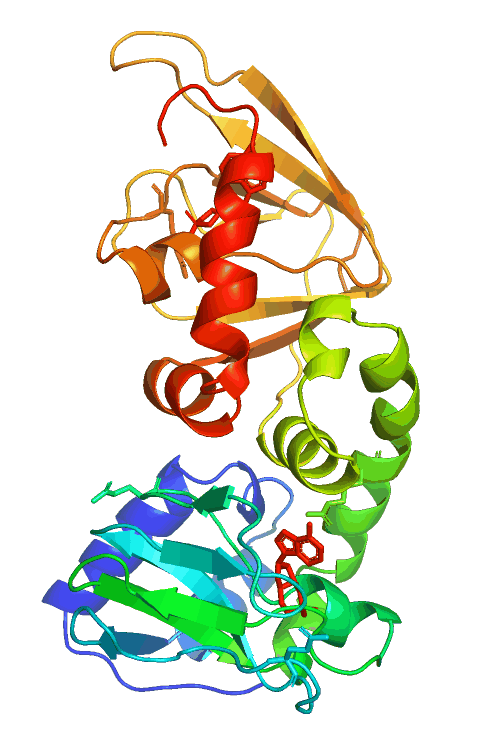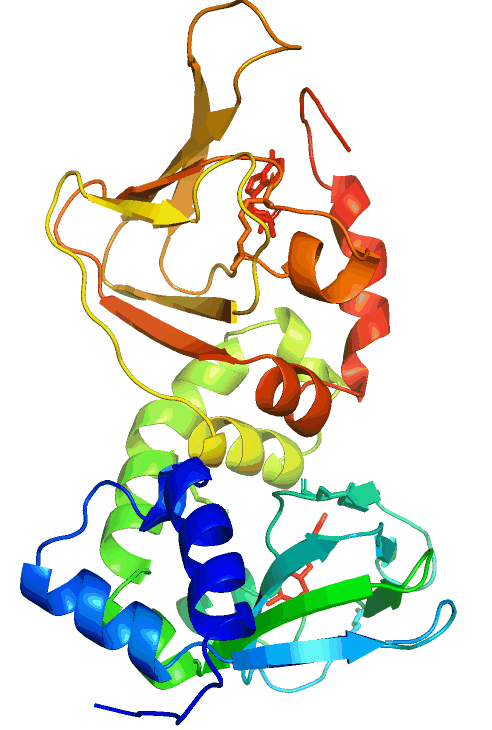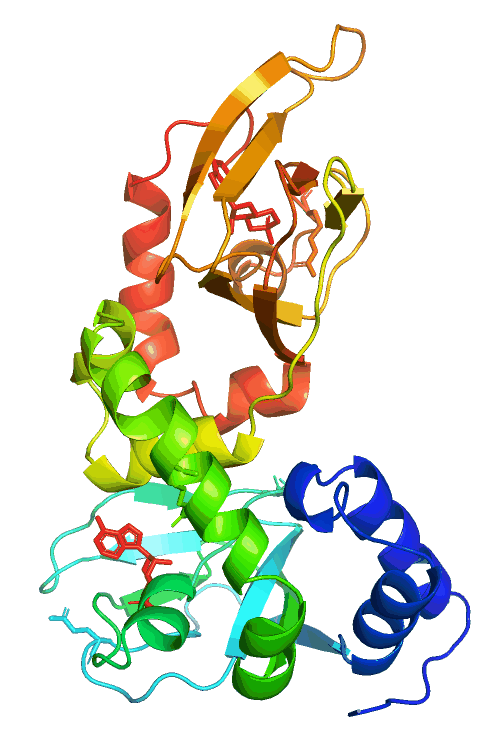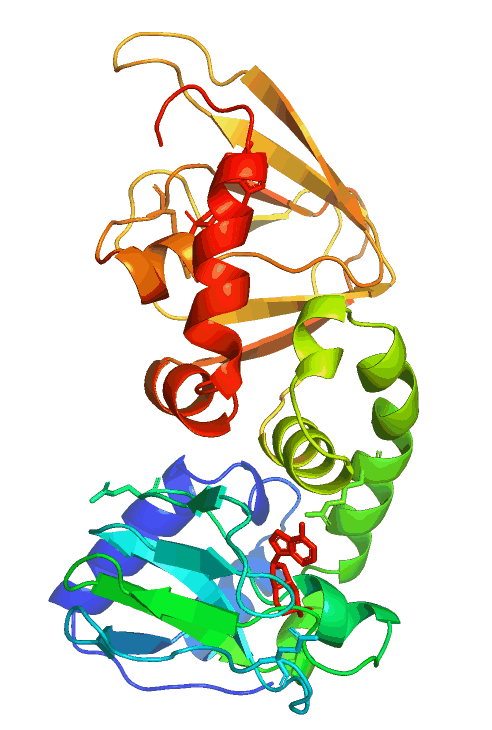
Anchored PKA signaling
Protein kinase A (PKA) is the main effector of the second messenger cAMP and plays critical roles in learning and memory. PKA is a heterotetramer of two regulary subunit and two catalylic subunits. Upon cAMP binding to the PKA regulatory subunits, the catalytic subunits are unleashed to phosphorylate protein substrates, including transcription factors and ion channels.
The subcellular localization and signaling specifity of PKA holoenzymes is determined by A-kinase anchoring proteins (AKAPs). The lab is particularly interested in A-kinase anchoring protein 1 (AKAP1), which targets PKA to the outer mitochondrial membrane to regulate mitochondrial dynamics (PMID: 21526220, 22049414, 30093535). Ongoing projects involve a recently generated conditional knock-out (cKO) mouse model of AKAP1, which allows us to the study the role of outer-mitochondrial PKA in specific cell types and brain regions.
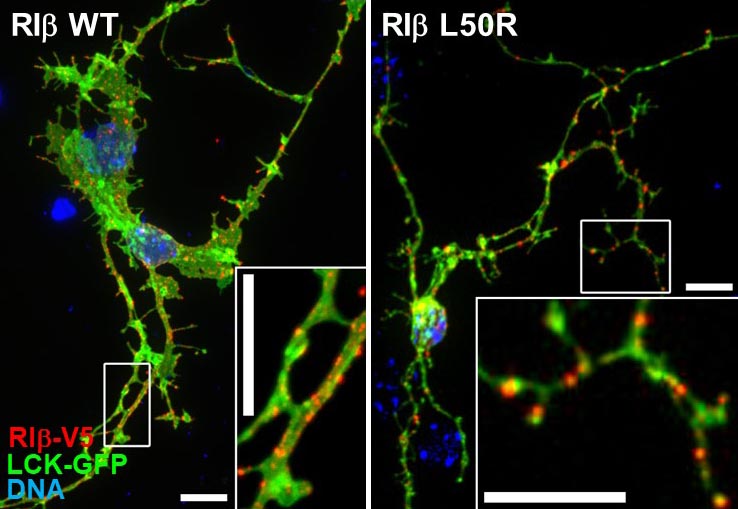
Pathological mutations in PRKAR1B (PKA/RIbeta)
Now routine diagnoses by next generation sequencing has enabled the identification of several mutations in the neuronal RIbeta (PRKAR1B) regulatory subunit. These mutations are either hereditary or de novo and cause a spectrum of neurological phenotypes.
The hereditary L50R mutation maps to the dimerization/docking (D/D) domain of PRKAR1B and leads to dementia and Parkinsonism with Lewy body-like inclusions in the brain. On the other hand, the R335W, R243C, and other variants map to or near the two cAMP-binding domains and cause neurodevelopmental symptoms, including intellectual disability with autism.
Together with collaborators at the U Iowa (Ted Abel, Marie Gaine) and UCSD (Susan Taylor, Jin Zhang), we are studying the effects of the mutations on PKA signaling in vitro and in vivo.